Glossary of Terms and Definitions For Bladesmiths
By Kevin R. Cashen, Master Smith
Ac1
The temperature at which austenite begins to form during heating. It is the arrest point on the iron carbon equilibrium diagram that corresponds with the boundary between the ferrite-cementite field and the fields containing ferrite and austenite or cementite and austenite. See also iron-carbon equilibrium diagram. The “c” (arreˆt chauffant) designates that it is a transformation point achieved by heating.
Accm
The temperature where cementite is completely dissolved in the austenite solution. It corresponds with the boundary between the cementite-austenite field and complete austenite in the hypereutectoid region of the iron carbon equilibrium diagram. See iron-carbon equilibrium diagram. The “c” (arreˆt chauffant) designates that it is a transformation point achieved by heating.
Ac2
See Currie point
Ac3
The temperature where ferrite is completely transformed to austenite solution. It is the boundary between the ferrite-austenite field and the complete austenite solution field, in the hypoeutectoid region, of the on the iron-carbon equilibrium diagram. The “c” (arreˆt chauffant) designates that it is a transformation point achieved by heating.
Arcm
The temperature at which cementite begins to form from the parent austenite solution during cooling of a hypereutectoid steel. The “r” (arreˆt refroidissant) designates that it is a transformation point achieved by cooling.
Ar1
The temperature where the transformation from austenite solution to ferrite and cementite (most often pearlite) is complete during cooling. The “r” (arreˆt refroidissant) designates that it is a transformation point achieved by cooling.
Ar3
The temperature at which austenite begins to transform to ferrite when during cooling. The “r” (arreˆt refroidissant) designates that it is a transformation point achieved by cooling.
AISI/SAE
These acronyms will most often be seen by knifemakers at the beginning of the designation of the steel they use, for example “AISI 1084”. They stand for the American Iron and Steel Institute and Society of Automotive Engineers. These organizations developed the numbering system that we used for identifying different alloys. In the AISI designation system the first two letters represent the alloying composition:
SERIES TYPES AND PERCENTAGE COMPOSITION. _
10XX Nonsulfurized carbon steels
11XX Resulfurized carbon steels (free machining)
12XX Rephosphorized & resulfurized carbon steels (free machining
13XX Manganese 1.75 (principal alloy)
23xX Nickel 3.50
25XX Nickel 5.00
31XX Nickel 1.25; chromium 0.65 (a binary alloy)
33XX Nickel 3.50: chromium 1.55 (a binary alloy)
40XX Molybdenum 0.20 or 0.25
41XX Chromium 0.50 or 0.95; molybdenum 0.12 or 0.20
43XX Nickel 1.80; chromium 0.50 or 0.80; molybdenum .25
44XX Molybdenum 0.40
45XX Molybdenum 0.52
46XX Nickel 1.80; molybdenum 0.25
47XX Nickel 1.05; chromium 0.45; molybdenum 0.20 or .35
48XX Nickel 3.50; molybdenum 0.25
50XX Chromium 0.25; 0.40 or 0.50
50XXX Carbon 1.00; chromium 0.50
51XX Chromium 0.80, 0.90, 0.95. or 1.00
51XXX Carbon 1.00; chromium 1.05
52XXX Carbon 1.00; chromium 1.45
81XX Chromium 0.80, 0.80, or 0.95; vanadium 0.12 (0.10 to 0.15)
81XX Nickel 0.30; chromium 0.40; molybdenum 0.12
86XX Nickel 0 55; chromium 0.50; molybdenum 0.20
87XX Nickel 0.55; chromium 0.50; molybdenum 0.25
68XX Nickel 0.55; chromium 0.50: molybdenum 0.35
92XX Manganese 0.85; silicon 2.00: chromium 0 or 0.35
93XX Nickel 3.25; chromium 1.20: molybdenum 0.12
94XX Nickel 0.45; chromium 0.40: molybdenum 0.12
98XX Nickel 1.00: chromium 0.80: molybdenum 0.25
The second two, and sometimes three, numbers represent the carbon content. For example, AISI 1080 is a simple carbon steel with less than 1 % manganese and approximately .80% carbon, AISI 52100 is a chromium alloy with 1.00% carbon.
Alpha iron
Iron that has a body centered cubic (bcc) atomic configuration and a much lower ability to dissolve carbon into solution. The natural state of iron at room temperature.
Alloy Steel
A steel with elements added to enhance certain properties. For example, 1060 is a simple carbon steel but 5160 is an alloy steel with chromium added for increased hardenability.
Annealing
The heat treatment for the softening and removal of the effects of strain in metal. Traditional full annealing for bladesmiths involves heating steel to above the recrystallization temperature and then slow cooling in an insulating medium such as vermiculite in order to form pearlite.
Austenite
The phase of steel that occurs at temperatures above 1333oF, or Ac1 on the iron-carbon equilibrium diagram, that is a solid solution of carbon in iron. Austenite is the condition of steel when forging occurs due to its very soft, malleable and ductile nature. Austenite is nonmagnetic and was named for British metallurgist Sir W.C. Roberts Austen. All the other phases that bladesmiths make with steel result from a transformation of austenite.
Austenitize:
To heat steel to the point that austenite is the primary phase of its makeup. Complete austenization would involve heating to a temperature in excess of Accm or A3 on the iron carbon equilibrium diagram.
Billet
A semi completed bar of steel smaller than a bloom from with is has been reduced, For bladesmiths a billet is most often used to refer to semi-finished bar of damascus steel that has yet to be forged into a final shape.
Bloom
A larger mass of raw steel or iron material that has yet to be forged into a billet. For bladesmiths that make their own steel from raw ore the bloom is the spongy mass taken from the smelting furnace that requires consolidation through forging.
Body centered cubic (bcc)
The unit cell in the atomic stacking configuration of iron that is shaped like a cube with an atom at each corner and one in the center. It is magnetic. Room temperature iron is bcc and carbon has very limited solubility in bcc iron.
Brine
A quenchant that consists of salt (NaCl) dissolved in water. The salt acts to defeat the vapor/steam jacket of the water to increase the quench effectiveness. Brine is a very extreme quench that can lead to cracking without very careful precautions.
Brittleness
The lack of ability a material to stretch or deform before fracture.
Carbide
In steel a compound of carbon with another alloying element. The most common is iron carbide (Fe3C) or cementite, but other elements in steel that form carbides include chromium, vanadium, tungsten, columbium and titanium. Carbides are much harder than the surrounding metal and their primary benefit is in increasing wear resistance but in small quantities can be a grain refiner.
Carbon steel
A steel with very limited alloying beyond its carbon content. 1060 is a simple carbon steel while 5160 is an alloy steel. This term is also often used in knife making to indicate that steel is not a stainless alloy.
Cast iron
A simple iron-carbon material containing greater than 2% carbon.
CCT diagram
Continuous cooling diagram. A diagram that shows the transformation products of austenite when cooled continuously, as opposed to the I-T (Isothermal Transformation) Diagram which shows transformation products at one temperature at equilibrium.
Cementite
The form that carbon takes in steel when it bonds with iron to form carbide (Fe3C). Cementite is very hard and brittle.
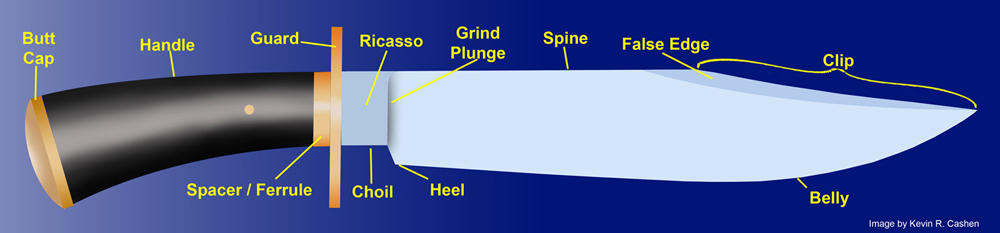
Choil
A recessed area on the bottom side of the blade directly in front of the guard before the edge, it often forms the bottom side of the ricasso. A choil is a natural result of drawing the edge down in forging and is useful in allowing complete access to the edge in sharpening.
Clinker
The slaggy masses that from as the unburnable silicates in coal concentrate in the bottom of the forge. Clinkers restrict the forge air blast and create a mess in the fire and on steel surface, so it is a bad fire practice to allow them to form in large sizes.
Clip
The areas on the spine of the blade near the tip that possesses a different angle than the rest of the spine, clips can often be sharpened or have a false edge.
Coke
The pure carbonaceous part of forging coal left after the volatiles have been burned out. Although coal forges burn coal, forging is only done in coke instead of the green coal which has sulfur and other contaminants which are detrimental to the steel.
Compressive Strength
The ability of a material to withstand compressive, or squeezing, type stresses.
Critical temperature
The temperature at which a change in crystal structure, phase or properties occurs. This can be any such temperature but is most often used by bladesmiths in reference to Ac1.
Currie point
The temperature at which a metal loses its ferromagnetic properties, for iron this is 1414oF. Also known as A2 in the iron-carbon equilibrium diagram.
Decalescence
The arrest in the rate of energy given off from a heated bar of steel as it transforms from one crystal state to austenite. On heating this transformation is endothermic in that it requires significantly more energy to complete and this creates a shadow effect in the steels incandescence.
Decarburization or “decarb”
Loss of carbon from the surface layer of a carbon bearing alloy due to reaction with the atmosphere it comes in contact with. Some degree of decarb is almost unavoidable in the forging operation but it can be kept to an inconsequential minimum with proper forge atmospheres and careful forging techniques.
Deep hardening steel
See “Shallow hardening steel”
Diffusion
The movement of constituent atoms to new positions within a material tending to make the composition of all parts uniform. The diffusion of carbon in steel brought about by heating is responsible for many of the changes bladesmiths rely on.
Distal taper
The gradual and continuous decrease in thickness of a blade moving from the guard to the tip. A blade without proper distil taper will lack balance and feel awkwardly heavy.
Drawing
As in “drawing a temper” it is a less accurate or less common term for tempering.
Ductility
The property of a steel that allows it to easily deform instead of resisting or breaking when stretched.
Eutectoid
The point in the carbon content of steel, approximately .8%, where all carbon can be in solution as the lowest temperature without excess iron or carbide, and transform completely to pearlite on cooling. AISI 1080 would be called a eutectoid steel due to its carbon content. The eutectoid should not be confused with the eutectic, which is a point of liquidus in cast irons above the carbon content of steel.
Face centered cubic (fcc)
The unit cell of the atomic stacking configuration of iron that is shaped like a cube with and atom at each corner and one in the center of each face. It is non-magnetic and is only stable at temperatures above 1335oF. Carbon has a very high solubility in fcc iron and austenite is a solution of carbon in fcc iron.
Fatigue strength
The ability of a material to withstand repeated loading.
Ferrite
The low carbon iron constituent of steel
Flexure strength
The ability of a material to handle gradual bending, usually involving tensile forces on one side and compressive forces on that other.
Forging
Forming of metal with dies, presses or hammers at high temperature.
Full tang
A handle attachment that has the tang of the blade exposed in a sandwich configuration between two handle slabs.
Fuller
A groove down the side of a blade for the purpose of lessening weight without sacrificing strength.
Fullering tool
Also often referred to as a “fuller” itself, this is a tool used in forging to pinch in a narrow area notch or grove in the work.
Gamma iron
Iron that has a face centered cubic (FCC) atomic configuration
Grain
An individual unit in steel having a single crystalline orientation. Most often grains are established with austenite and the other phases forming from or with the austenite grains. To observe grains without metallographic equipment a bladesmith must break the steel and observe the outside surfaces of the grains in the fractured surface. Individual grains that can be resolved with the naked eye are far too large for a good blade. Grain size is kept fine through careful control of heat, and proper normalizing.
Hamon
Pronounced “hah-moan”, this is a Japanese term for the decorative hardening line created by differential quenching methods most often using the application of clay.
Hardening
The heat treatment consisting of heating a blade to put carbon into solution to make austenite and then quenching it to lock the carbon in place to make the hardened steel phase of martensite.
Hardness
A measure of the steels strength, or ability to withstand deformation, a hard steel can be brittle but is not ductile.
Hidden tang
A handle attachment that involves a narrow tang hidden within the handle material.
HRC
Hardness Rockwell “C” scale or HRC is the label added to a number derived from that test for the hardness of steel. A hardened blade may measure from 57HRC to 63HRC or more. The label is necessary because there are other Rockwell scales (A, B etc…) and without the specifying the scale the numbers have no meaning.
Hypereutectoid
A steel that has a carbon content greater than .8% (the eutectoid). A hypereutectoid steel will form austenite with leftover carbide that requires greater heat to dissolve and pearlite with leftover carbide when cooled from solution. 1095, W2 and 52100 are examples of hypereutectoid steels
Hypoeutectoid
A steel that has a carbon content less than .8% (the eutectoid). A hypoeutectoid steel will form complete austenite more slowly due to the excess iron (ferrite) for the amount of carbon. When cooled from solution a hypoeutectoid steel will form pearlite and leftover ferrite. 1050 and 5160 are examples of hypoeutectoid steels.
High temperature salt bath
The part of a salt bath heat treating system that uses molten salts at high temperatures (1275oF and above) for heating the steel in preparation for quenching. High temperatures salts are often neutralized sodium chloride based but can have other heavy metal additions in order to handle higher temperatures.
Impact strength
The ability of a material to withstand shock or sudden loading.
Interstitial alloying
Alloying elements with atoms that are smaller than the solvent atoms in the metallic solid solution, and because of their size occupy spaces between the larger atoms. Carbon is the most obvious example of an interstitial alloy element.
Interrupted quenching
An improvised method of approximating a marquench by interrupting a traditional liquid quench just above the Ms point and allowing the blade to complete the quench with air cooling.
Iron-Carbon equilibrium diagram
(iron-iron carbide equilibrium diagram, iron-carbon phase diagram) A chart that shows the phases and solubility of carbon in iron based on percentages of carbon versus temperature. The part that of the diagram that concerns knifemakers rests to the far left, below the 2% carbon level where hypoeutectoid, eutectoid and hypereutectoid steels are involved. This diagram is based on iron and carbon only and does not reflect the effects of additional alloying or non-equilibrium conditions.
I-T Diagram
The I-T diagram, or isothermal transformation diagram, also known as a “TTT curve” (time-temperature-transformation curve) is a chart which shows the various phases that a steel will transform to when heated to a given critical temperature to create austenite and then cooled to subsequently lower temperatures. Unlike the Iron-Carbon equilibrium diagram the I-T diagram is specific to each distinct steel chemistry so that every alloy will have its own unique curve.
Lamellar anneal
An annealing process with the specific goal of creating coarse pearlite. For most bladesmiths it consists of heating above non-magnetic and then insulating the blade in wood ash or vermiculite for a slow cool.
Low temperature salt bath
The part of a salt bath heat treating system that holds lower temperature (300oF to 700oF) molten salts to be used as a quenching medium. Low temperature salts are often mixtures of potassium and sodium nitrates and nitrates made specifically for their working rage and cooling properties.
Malleability
The ability of a metal to be hammered into different shapes without breaking.
Marquenching
The quenching process that involves rapid cooling in a liquid medium to a temperature just above the point martensite begins to form (Ms) where the cooling is arrested to allow the steel temperature to equalize followed by air cooling.
Martensite
The phase present in hardened steel. Martensite is a distortion of the normal body centered atomic stacking of iron in room temperature steel into a body centered tetragonal configuration due to carbon atoms trapped within the matrix. Martensite was named for the German metallurgist Adolph Martens, and has a very distinct acicular (needle like) appearance under the microscope.
Ms
“martensite start”. Where steel actually begins to harden in a quench, it is the point on cooling austenite when a sufficient low temperature has been reached for the initiation of the diffusionless shear type transformation that results in martensite. The martensite start point, for many of the simpler steels used in bladesmithing, often falls within a range from 400oF. to 500oF. The martensite start point is designated Ms on charts such as I-T or TTT curves.
Mf
“martensite finish”. The point on cooling when the diffusionless shear type transformation that results in martensite is complete, and maximum martensite conversion is achieved. Certain alloys and circumstances can stabilize austenite causing Mf to actually fall below room temperature, resulting in retained austenite. The martensite start point, as designated Mf on charts such as I-T or TTT curves in the past. is now more commonly replaced with a maximum percentage such as M90%.
Neutral flame
A flame, or forge atmosphere, that has neither excess oxygen nor fuel.
Normalizing
Often confused with annealing but a distinctly different heat treatment aimed at bringing about a more homogeneous condition inside the steel. For bladesmiths normalizing consists of heating the steel as evenly as possible to a temperature to affect a desired change in carbide, austenite grain, or strain, followed by an air cool. Industry most often uses much higher temperatures for normalizing than bladesmiths, and often avoids normalizing steels that may air hardening to any degree. But for the effects of carbide and grain condition on cutting edges almost any simple steel that bladesmiths work with will benefit from normalizing.
Oxidizing flame
A flame, or forge atmosphere, that has an excess of oxygen for available fuel.
Partial tang
A handle attachment that involves a narrow tang that only extends partially into the handle material and not passing all the way through.
Pearlite
The steel phase that consists of alternating lamellae of iron (ferrite) and carbide (cementite) allowed to separate from solution by slow cooling. Pearlite gets its name from its mother of pearl appearance under the microscope. The coarseness, and corresponding levels of softness of pearlite is determined by the rate of cooling. Normalizing, cooling in air produces fine pearlite, while annealing, slow cooling in an insulating medium produces coarse pearlite.
Penetration hardness
The hardness value obtained by measuring a materials resistance to being indented by a penetrator. Rockwell, Vickers and Brinell are examples of penetrative hardness tests..
Profile taper
The change in blade width from the guard to the point. On many double edge blades it decreases distally for balance and point control, but on some large chopping it can be the reverse for mass distribution in cutting.
Quenching
The rapid super-cooling of austenitized steel for the purpose of hardening. Quenching requires a quenching medium (quenchant) that matches the needs of the specific steel chemistry. Quenching mediums can include brine, water, oils, air and molten salts among others.
Rc
Another way of designating the Rockwell “C” scale. See also “HRC”.
Recalescence
The liberation of excess energy when steel undergoes the transformation from austenite to another phase on cooling. This transformation is an exothermic reaction, and gives off more energy than the surrounding untransformed material creating a brighter region in the steels fading incandescence.
Recovery
The reduction or removal of strain hardening (cold working) effects before the point of recrystallization during heating.
Recrystallization
The change from one crystal structure to another in heating or cooling.
Reducing flame
A flame, or forge atmosphere, that has an excess of fuel for available oxygen.
Ricasso
A squared area of the blade directly in front of the guard that has no edge.
Rockwell
A test for measuring hardness that consists of a device that very precisely forces a penetrator into the steel surface under a minor load, to set a baseline, followed by a major load. The depth of penetration between these two loads is then very accurately measured and assigned a numerical value. The Rockwell test is the most common hardness test used in the United States and its method of reading eliminates much of the error in human interpretation for results. There are differing scales (A,B,C and D) with corresponding penetrators and load values for different materials. Hardened steel is measured mostly with the “C’ scale.
Salt baths
A heat treating system that involves the use of molten metal salts for the purpose of heating and cooling the steel.
Scratch hardness
A measure of a materials ability to withstand scratching in a quick check with a tool such as a scribe or file. The file check and the Moh hardness test are examples of scratch hardness tests.
Shallow Hardening Steel
This term most likely has its origin in the Jominy end quench test. In this procedure a round steel specimen is austenitized and then suspended vertically over a jet of water that super cools the end. Hardness (HRC) readings are then taken at regular intervals to determine the depth and degree of hardening. From this a hardenability curve is created for the tested steel. The same affect can be seen by cross sectioning any hardened piece of steel and testing the hardness from its outer surface to the center of the piece. Steels having low hardenability, and requiring faster quench speeds are thus said to be “shallow hardening”. Steels that easily reach higher HRC values farther from the quenched surface are said to be “deep hardening”. Many simple carbon steels (1060, 1084, 1095 and the “W” tool steels”) lack alloying elements that promote deeper hardening, and are thus shallow hardening when compared to alloy steels.
Snap temper
A quick, or low temperature, stress relieving treatment applied to fully hardened to prevent distortion or cracking when there will be a delay in an actual full temper.
Soak time
The time steel is held at a given temperature to allow for the effects of the desired thermal treatment. It is most often used to refer to the time a blade is held at temperature before quenching in order to put sufficient carbon into solution for proper hardening. Simpler steels have less soak requirements, but this changes with the increase in additional alloying.
Solid solution
A mixture of elements or compounds where each maintain their own properties but in an overall solid form. In any solution there will be a solvent that dissolves or hosts a solute which is dissolved. In steel iron is the solvent and carbon is the solute.
Spark Test: A quick precursory test that can be performed in almost any shop that relies on the concept that various chemistries of different steels will display a spark stream of different color, shape or intensity, when ground.
Spheroidizing
The heat treatment designed to soften or anneal steel by heating to temperatures below full recrystallization and taking carbon out of solution by forming spheroidal carbides in a soft iron matrix. Spheroidized is the standard condition that most steel stock will be in as it arrives from the mill and is the best approach for any steel that must be machined and particularly better for steels with .8% carbon content or higher.
Strain
A measure of the relative deformation of a material when force is applied to it.
Stress
The measure per unit area of the force applied to a material.
Stress relieving
The heat treatment used to relieve any strain effects from heavy machining and other cold working applications. Stress relieving in simple steels consists of heating to around 1200oF and air cooling.
Substitutional alloying
Alloying elements with atoms that are larger than the solvent atoms in the metallic solid solution and because of their size can only replace an atom while occupying its space in the atomic arrangement. Chromium would be an example of a substitutional alloying element.
Tang
The portion of a blade that is covered by or is used for attaching the handle
Tempering
The heat treatment performed on hardened steel to reduce brittleness and increase toughness. For most steels that bladesmiths use tempering temperatures will range from 350oF. to 500oF. The mechanism by which tempering works is in allowing very limited movement (diffusion) of the carbon atoms trapped in the body centered tetragonal martensite to permit a relaxation of the distortion to a more stable body centered atomic stacking. The liberated carbon then forms submicroscopic tempering carbides which will grow as temperature is increased and overall hardness decreases.
Toughness
The ability of steel to handle sudden loading without failure.
Young’s Modulus
Also known as modulus of elasticity, it is the values obtained when a load or stress is applied to a metal, within its elastic range, to stretch it to a given length. For steel this is approximately 30,000 PSI for .001″. This will be the case regardless of the heat treatment.
